Anabolic Steroid Induced Hypogonadism (ASIH) & Treatment
Introduction
Anabolic steroid induced hypogonadism (ASIH) is the functional incompetence of the testes with subnormal or impaired production of testosterone and/or spermatozoa due to administration of androgens or anabolic steroids. Nonprescription and prescription AAS use account for more than four million males taking AAS in one form or another for a limited duration. While both uses deal with the effects of AAS administration they do not account for the period after AAS cessation. Both prescription and nonprescription, use is a cause of ASIH. The signs and symptoms of ASIH directly impact the observation of an increase in muscle mass, muscle strength, well-being, and more from AAS administration. ASIH is critical towards any future planned use of AAS or similar compound.
AAS have been prescribed for cachexia associated with human immunodeficiency virus, cancer, burns, renal and hepatic failure, and anemia associated with leukemia or kidney failure. More significantly, upon AAS cessation the comorbid condition of hypogonadism adds to their already existing chronic illness potentially significantly worsening their health. The further understanding and treatments that mitigate or prevent ASIH could contribute to therapies for these conditions and more, particularly obesity, stopping nonprescription and prescription AAS use, and restoration of the HPTA. The nonprescription use of AAS paved the way for ways to treat ASIH. To the uninitiated medical professional this is termed post cycle therapy [PCT]. In this brief review, ASIH history, diagnosis, and treatment is discussed. Finally, future areas of research are proposed.
It has been almost 20 years since I first published studies on the treatment of ASIH. [1-4] I have had to make a great personal sacrifice in this clinical endeavor. Many will be familiar with my story and the fight for the proper medical care of the AAS user. More than anything, this impressed upon me the politicization of AAS that continues to this day. And the fear, contempt, and condescension of the medical community towards the AAS user. Add to this that the educated AAS user knows much more than almost all physicians. Find Waldo! The irony is overwhelming from this commentary just written this year: [5]
To restore ASIH, treatment strategies that increase the production of endogenous testosterone through treatment such as testosterone replacement, SERMs, and hCG should be done through accurate diagnosis and consultation with medical professionals. … On the one hand, clinicians should have sufficient knowledge of AAS use, concepts of ASIH, and treatment strategies.
This is unacceptable. In the interim there have not been a study detailing the treatment of ASIH other than a conceptual proposal for treatment almost 5 years ago. And, even here there has been no follow up on the proposed treatment. This conceptual proposal by Rahnema et al. is instructive for treating ASIH and will be further commented on following. [6]The study by Rahnema et al. highlighted a surprising issue in the understanding of AAS use in that no studies on the subject met inclusion criteria to perform a meta-analysis. A systematic review of the literature over a 48-year period from 1965 to 2013 and of expert opinion was performed. Of note, Rahnema et al. did cite the article by Tan and me that included multiple references on ASIH treatment. [7]
This systematic review provided an educational insight into the behavior of AAS users and medical professionals and the side effects, including infertility, with subsequent management including ASIH. [8]
The dilemma that always exists for providers is whether treating such patients, and the sudden or later hypogonadism, is feeding into their abuse/dependent personality versus a liberal and nonjudgmental belief that these men need help with their physical symptoms and signs. Rahnema et al. proposed an example of a ‘‘recovery’’ protocol for such men.
With multiple different AAS regimens being used and variable durations and cycles of treatment, the exact recovery from AAS is not known. A prospective study of different real world AAS regimens and recovery would be difficult to conduct in a structured and ethical manner since these are controlled drugs that are obtained illicitly from a number of questionable sources of variable quality and given in doses that are much higher than are considered safe. Trials of different recovery medication protocols and subsequent expert opinion may remain the only methods to study the treatment of these men after AAS abuse.
The development of AAS compounds originally were originally for treatment of hypogonadal dysfunction and commencement of delayed puberty in men and for growth promotion. [9] Moreover, scientific and official court documents, including doctoral theses and scientific reports, demonstrate the positive effects of these and other hormonal drugs on muscle strength and performance in elite sports were common knowledge and had been in practice since the early 1960s. [10] Due to their anabolic effects, AAS became vastly popular among athletes.
Controversy raged for decades over the effectiveness of AAS in promoting muscle mass and muscle strength. Despite the admitted illicit use of AAS by athletes, the record breaking in Olympic events, the obvious appearance in musculature enhancement, and more the medical and research community disputed and denied the AAS effects. [11] After a considerable period of scientific controversy, it is now clear that anabolic-androgenic steroid hormones are effective in increasing both muscle mass and muscle strength.
Another of the held beliefs by the medical community deals with the period after anabolic steroid cessation, not their administration. The prevailing medical opinion is that clinically significant ASIH occurs from nonprescription AAS use but not from clinically prescribed AAS. Male contraceptive studies clearly demonstrate this not to be the case. The evidence points to hypogonadism as a potential consequence of AAS use and appears dependent on dose, duration, and type of AAS used.
Hypothalamic Pituitary Testicular Axis [HPTA]
The HPTA is a dynamic feedback loop. Structural components of the HPTA are the hypothalamo-pituitary, testicles, and androgen receptor (AR) located on certain end organs (prostate, bone, and muscle). The major hormones of the hypothalamic pituitary testicular axis are gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), inhibin, testosterone (T), dihydrotestosterone (DHT), and estradiol (E2). [12]
Pituitary secretion of LH and FSH is controlled by the pulsatile secretion of GnRH, the secretory products of the testes (testosterone and inhibin), and metabolism of testosterone (estradiol). In males, luteinizing hormone (LH) secretion by the pituitary positively stimulates testicular testosterone (T) production. Regulation of the secretion of GnRH and LH is by the negative feedback of testosterone and estradiol at the level of the hypothalamo-pituitary.
Although testosterone is the major steroid secreted by the testis, it has been long recognized that estradiol is involved in the regulation of gonadotropin secretion. The plasma estradiol mainly arises from aromatization of testosterone. Estradiol has a much larger, inhibitory effect than testosterone, being 200-fold more effective in suppressing LH secretion. 5?-reduction, DHT, does not appear to play a significant role in the negative feedback effect. In fact, much of feedback inhibition of LH secretion by testosterone can be accounted for by its bioconversion to estradiol. The role of GnRH and E2 in HPTA regulation comes into play for ASIH treatments. [13-15]
Of note, the suppression of FSH and LH by AAS are disparate. These findings provide the basis for the current understanding of the dual control of the endocrine and spermatogenic functions of the testes by LH (via production of testosterone) and FSH (via production of inhibin). Inhibin from Sertoli cells of the testicles has a negative feedback on FSH secretion. This relationship between inhibin and FSH is observed across the physiological range in normal men. This strongly suggests that inhibin is an important component of the afferent arm of the feedback loop from the testis, selectively regulating FSH secretion.
[Note: The master regulator of the male gonadal axis was long thought to be the GnRH neuron, but recent investigations now implicate upstream kisspeptin, neurokinin B, and the endogenous opioid peptide, dynorphin (KNDy) neurons as the master regulator, and have better defined how KNDy neurons are regulated by steroidal and nonsteroidal signals.]
Anabolic Steroid Induced Hypogonadism [ASIH]
For close to fifty years,published literature extensively demonstrates hypogonadism occurring after AAS cessation. [16-29] Consistently, there is found a dramatic suppression of serum gonadotropins and testosterone levels that continues for an indefinite period after AAS cessation. Published literature uniformly finds AAS administration, both prescription and nonprescription, induces a state of hypogonadism after AAS cessation. All compounds classified as AAS cause a negative feedback inhibition of the hypothalamic pituitary testicular axis, suppress endogenous gonadotropin secretion, and as a consequence endogenous testosterone production.
An unproven and unfounded assumption has been made in the medical establishment that the treatment for an individual suffering from ASIH is to do nothing which is coined ‘watchful waiting’ and in time HPTA functioning will return to normal. This premise can be traced back to Knuth et al. (1989) studying semen parameters in AAS users. He concluded, “Results suggest that even after prolonged use of extremely high doses of anabolic steroids, sperm production may return to normal.” [30]
The effects on testes and sperm production are due to AAS induced suppression of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels. The return of normal FSH levels typically precede LH levels after cessation of AAS, including testosterone. However, spermatogenesis returns in the presence of hypogonadal testosterone levels. In male contraceptive studies, recovery without medical therapy to stimulate endogenous testicular function is slow. The best available data come from a 2006 integrated multivariate analysis of 30 previously published clinical trials, which reported that the probabilities of recovery to 20 million sperm/mL at 6, 12, and 24 months were 67%, 90%, and 100%, respectively. [31] The return of spermatogenesis is not the equivalent of a return of normal serum testosterone levels.
Demonstration that the return of spermatogenesis does not equate with the return of normal serum testosterone is clear from a study on assisted reproduction. In 2003, there was a reported case study of a male patient with azoospermia receiving the prescription androgens testosterone enanthate and oxandrolone, undergoing assisted reproduction. After discontinuation of both androgens the serum testosterone level, 134-ng/dL, was still well below the normal range (270-1100 ng/dL) but with normal sperm count of 63 X 10^6/ml. This is the identical clinical situation described above for the return of spermatogenesis but not the serum testosterone.[32]
The academic and medical communities have erroneously assumed that only the use of large AAS doses and simultaneous multiple AAS use for a prolonged duration results in hypogonadism after AAS cessation. The belief, unsupported and unsubstantiated, is prescription AAS would give no reason for concern. After AAS administration, HPTA suppression follows, with the variables being the duration and severity. The publication of dose-response relationship studies between AAS administration, AAS cessation, and HPTA normalization have yet to occur.
Selective Androgen Receptor Modulators (SARM)
Nonsteroidal androgen or selective androgen receptor modulators (SARM) administration is currently in the research and investigational stages. These studies indicate that their clinical use will result in induced hypogonadism after cessation by their effects on gonadotropin levels This same opinion was voiced by investigators, ‘‘Selectivity with regard to gonadotropin suppression represents a significant barrier to the clinical use of SARMs” [33-34]
In particular, LGD-4033 administration was associated with dose-dependent suppression of total testosterone, sex hormone–binding globulin, high density lipoprotein cholesterol, and triglyceride levels. follicle-stimulating hormone and free testosterone showed significant suppression. [35] Despite these failings, LGD-4033 originally developed by Ligand Pharmaceuticals has been licensed by VKTX as VK5211 as a treatment for recovery from nonelective hip fracture surgery. [36] This will fail.[Note: SARM are worthless/harmful for/in PCT use.]
Treatment & Post Cycle Therapy (PCT)
The armamentarium of available therapies includes agents that promote pituitary gonadotropin production by reducing estrogen receptor-mediated negative feedback (selective estrogen receptor modulators such as clomiphene citrate and tamoxifen, aromatase inhibitors such as anastrazole and letrozole), gonadotropin replacement therapy with human chorionic gonadotropin (hCG, bioequivalent to LH and recombinant or urine-derived FSH), and GnRH receptor agonists. [37]
The return of HPTA functionality and restoration is brought about using combined therapy or direct pituitary stimulation. There are many variations of these themes and no one treatment has been shown to result in a successful restoration 100% of the time. The best evidence published to date are the case and observational studies. As before, the nonprescription AAS user is leading the way on the exploration of treatments aimed HPTA restoration. [37]
human Chorionic Gonadotropin (hCG)
Human chorionic gonadotropin (hCG) is a polypeptide hormone produced by the human placenta, is composed of an alpha and a beta sub-unit. The alpha sub-unit is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH). The beta sub-units of these hormones differ in amino acid sequence. [38]
Its use has proven beneficial in many clinical situations including stimulation of testosterone and sperm production. [39-41] hCG has been shown to significantly improve gonadal function in hypogonadotropic hypogonadal adult males. hCG’s effect is centralized at the Leydig cells of the testicles and stimulates hormone function at the testicular level but does not reverse hypothalamic-pituitary suppression. For this reason, hCG use alone is problematic for HPTA restoration.
In 1998, a case study reported on the use of hCG successfully in ASIH. [42] A young competitive body-builder who abused anabolic steroid drugs and developed profound symptomatic hypogonadotrophic hypogonadism. With the help of prescribed testosterone (Sustanon) he stopped taking anabolic drugs, and later stopped Sustanon also. Hypogonadism returned, but was successfully treated with weekly injections of human chorionic gonadotropin for three months. Testicular function remained normal thereafter on no treatment. The author concluded, “The use of human chorionic gonadotropin should be considered in prolonged hypogonadotrophic hypogonadism due to anabolic steroid abuse.”
Noteworthy, doses ranging from 750 to 1500 IU of hCG, divided into one to three weekly doses, are necessary to restore and maintain normal testosterone serum levels in hypogonadotropic hypogonadic male subjects. This illustrates the large intersubject variability and highlights the caution in extrapolating the results and simulations from healthy (albeit down-regulated) subjects to hypogonadic patients. [43] Currently, I utilize hCG 1,000-2,000 IU every three days during the period or longer the AAS clear.
Extractive (also termed urinary) [ehCG] and recombinant [rhCG] hCG are virtually identical to that of pituitary LH, although ehCG appears to have a small degree of FSH activity as well. FSH activity appears not to be present with rhCG. Pituitary, pulsatile LH production results in a ?90 min half-life while ehCG, has a long half-life (24+ hours). hCG is water soluble and sold as a sterile lyophilized powder. When reconstituted with bacteriostatic water for injection preserved with benzyl alcohol 0.9%, the solution should be refrigerated and used within 30 days, although it has been found to be effective for longer periods.
[Note: Claims that oral or sublingual HCG are as effective as when hCG is given by injection are marketing attempts intended to deceive scientifically unsophisticated consumers. As added proof of its effectiveness, people point to a patent(s). Bottom line, the only effective and safe way to receive the benefits of hCG is by injection. And, there is no documented case of primary hypogonadism because of hCG.]
[Note: Several natural and recombinant preparations of gonadotropins are currently available for therapeutic purposes. The difference between natural and the currently available recombinant preparations (which are massively produced in Chinese hamster ovary cells for commercial purposes) mainly lies in the abundance of some of the carbohydrates that conform the complex glycans attached to the protein core.] [44]
Selective-Estrogen Receptor-Modulators (SERM) & Aromatase Inhibitors (AI)
Estradiol is a prime target for HPTA restoration since it plays such an important role in HPTA regulation. This can be done by blocking the receptor through the action of a selective-estrogen receptor-modulators (SERM) [sometimes called anti-estrogens) or decreasing the available estradiol by blocking its synthesis from testosterone with aromatase inhibitors (AI). [45] In either case, the effect at the CNS level results in a significant increase of gonadotropin levels. In turn, LH stimulates Leydig cells in the testes, and this leads to increased local testosterone production.
Selective estrogen receptor modulators (SERM) is a compound that can act as an estrogen agonist or antagonist, depending on the specific target tissue. At present, four SERMs are approved for clinical use: clomiphene, raloxifene, tamoxifen, and toremifene. Most of the unique pharmacology of SERMs can be explained by three interactive mechanisms: [1] differential estrogen-receptor expression in a given target tissue, [2] differential estrogen-receptor conformation on ligand binding, and [3] differential binding to the estrogen receptor of coregulator proteins. [46-49] There are a number of reported cases using SERMs for infertility and hypogonadism, including ASIH. [50-55]
[Note: Enclomiphene is an experimental SERM that appears to be available from certain sources. I question whether it is truly enclomiphene.] [56-57]
Aromatase is a cytochrome P-450 enzyme concentrated in the testes, liver, brain, and adipose tissue and is responsible for the conversion of T to E2. The currently approved AIs all powerfully inhibit estrogen synthesis, they may be subdivided into steroidal and nonsteroidal inhibitors, which interact with the aromatase enzyme differently. Nonsteroidal AI, letrozole and anastrozole, bind noncovalently and reversibly to the aromatase protein, whereas steroidal AI, exemestane, may bind covalently and irreversibly to the aromatase enzyme. Although aromatase inhibition by letrozole and anastrozole is close to 100%, their administration does not completely suppress E2 levels in men. [58-64]
Gonadotropin Releasing Hormone (GnRH)
Gonadotropin-releasing hormone causes the pituitary gland in the brain to make and secrete the hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Also called GnRH, LH-RH, LHRH, and luteinizing hormone-releasing hormone. Reported cases using GnRH stimulation have been few. If treatment with hCG and or SERM/AI is unsuccessful, there is little to lose in trying this route. In the treatment of ASIH, care must be taken with respect to dosing.
GnRH agonists (e.g., goserelin, triptorelin, buserelin and leuprolide) are decapeptides, with similar structure to native GnRH and a great affinity to the GnRH receptors. After their binding to the receptors on gonadotropes, they initially cause gonadotropin release (flare-up effect). With continuous administration, this is followed by a dramatic drop in the circulating concentrations of FSH and LH, through a desensitization mechanism. GnRH agonists have greater affinity for the GnRHR than native GnRH; they also have greater resistance to enzymatic breakdown and a prolonged half-life compared to native GnRH. There have been reports treating ASIH with Triptorelin, Gonadorelin, and Buserelin. [65-67]
Clinical Experience
It bears repeating that there is no single treatment for ASIH. For controlled studies on ASIH, there will need to be a change in the manner that AAS users are treated. Also, unfortunately, physicians see ASIH as more of an opportunity as an income source. This might be partly attributable to fear of retribution by medical boards. Owing to the negative feedback regulation of the HPTA provides for a step wise restoration in such a manner as to not finish with HPTA suppression. It should be noted, however, that recovery is often done using SERM or AI alone. The use of hCG alone as shown above is the rare exception.
The first phase of the HPTA protocol examines the functionality of the testicles by the direct action of hCG. An important consideration in the hCG stimulation test is the proper administration for both the dose and duration of hCG. This initial value is a measure of the ability of the testicles to respond to stimulation from the hCG. I administer hGG 1,000-2,000 IU E3D until the AAS clear or longer. I use a longer half-life to ensure clearance. In the original publications hCG and SERM are given at the same time. This was for patient compliance and is not necessary. A one-week overlap is enough to allow for adequate serum SERM once the hCG is stopped.
The second phase of the HPTA protocol examines the ability of the hypothalamo-pituitary to respond to stimulation by producing LH levels within the normal reference range. Long-term follow-up is necessary to ensure permanent reversal of hypogonadotropic hypogonadal conditions. Currently, I utilize variations of clomiphene, tamoxifen, or both. The most common being clomiphene 50 mg BID along with tamoxifen 20 mg BID. Four weeks off all meds provides an excellent idea for HPTA Restoration. If the LH is still high, one might wait a few more weeks to ensure HPTA function.
Commentary
Following is commentary on the article by Rahnema et al. and some aspects of ASIH treatment. Rahnema detailed in their publication a proposed a treatment for ASIH and shown below in the image. It is to be highlighted that this is a proposal and without any clinical data for support despite years passing.
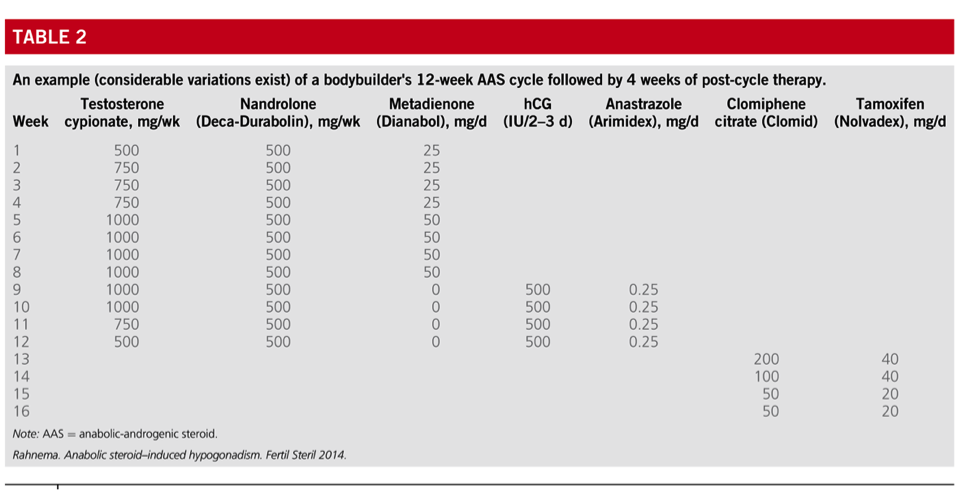
Also, they provided an example a12-week cycle. Cycle: The cycle is between 1-1.5 grams divided between testosterone cypionate [TC] and nandrolone decanoate [ND] for 12 weeks and Dianabol 25-50 mg ED for the first 8 weeks. hCG 500 IU E2-3D along with Arimidex 0.25 mg ED is added for the last 4 weeks. SERM are used from week 13-16 in decreasing manner: Clomiphene 200/100/50/50 mg ED, Tamoxifen 40/40/20/20 mg ED.
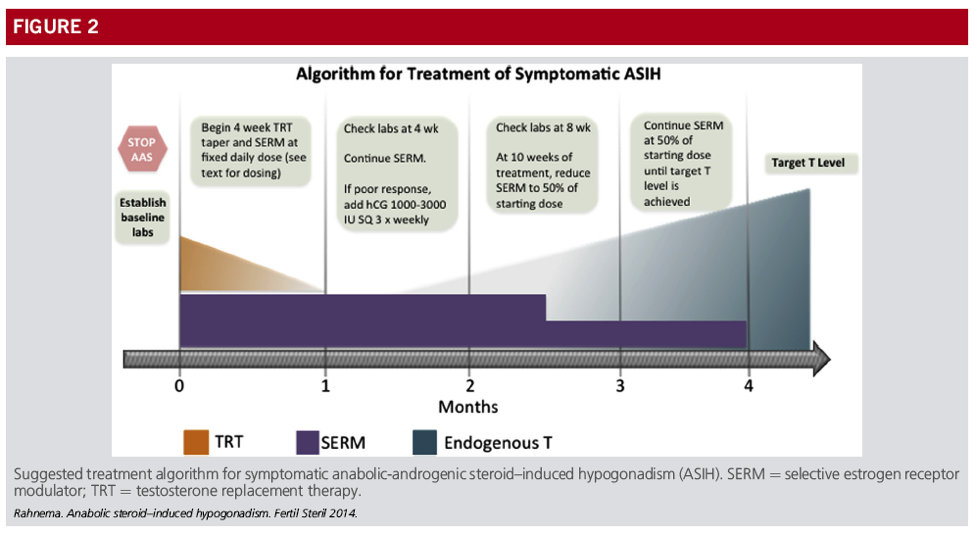
Taper: In the ASIH algorithm the taper is alongside the use of SERM. I would imagine the thinking for this is as the T level decreases and the HPTA becomes sensitive to the SERM the T levels will return to an acceptable level. But, as the image shows if the T level is not adequate, hCG is incorporated. In that case, the use of the SERM was of no consequence and the protocol must be essentially redone.
On a separate but similar topic, there are physicians that employ TRT tapering with goal the HPTA recovers. Notwithstanding, the T half-life serves as its on taper. If tapering was a real phenomenon, then TRT doses could be able to be tailored to the baseline serum testosterone. As you are undoubtedly aware, this is not the case.
A post-hoc analysis investigated the effects of endogenous testosterone (T) production reflected by baseline testosterone levels on testosterone replacement therapy (TRT) in hypogonadal men. [68] While baseline T levels may affect the final T concentration, a similar proportion of patients was within the normal range and reach the therapeutic goal. Patients, especially those with very low levels at baseline, may benefit from close monitoring of testosterone levels and appropriate dose adjustment. In other words, the HPTA was equally suppressed in both populations! Or, there would be a significant difference in TRT dose.
Another approach used is the incorporation of short acting esters at the end of the cycle to both maintain AAS levels and decrease the time to initiate SERM. This approach is in theory only. Until and unless the proper studies are done to check on this theory, this lessens the time of hCG use. There is no evidence this will for a faster recovery and might delay recovery in failing to bring the testes online enough through hCG.
AAS Clearance: The 2-week rule used by many is to start SERM 2 weeks after the last pin regardless of the cycle. Other than no PCT this is the number one reason I see for ASIH. Common sense would tell one that using 2 weeks before using SERM can not be right for different cycles. But, it is commonly found on forums.
As an example, the Bhasin 1996 NEJM study with TE 600 mg showed TT levels up to ~3500+ ng/dL one week after the last pin. [Not an uncommon cycle dose.] If a half-life of ~7 days is used, it will take ~4 weeks to have T levels <300. The point at which the pituitary would be open to stimulation. It might be less. Again, the error is too quick, not too slow for HPTA functionality/restoration. [69]
In the AAS cycle example (Table 2), they go further and have no time between the last AAS and SERM initiation. The total AAS dose is one gram [and includes the suppressive nandrolone decanoate). Those familiar with the half-lives as well as the suppressive effects of ND will recognize that the AAS will not be cleared enough from the body for the HPTA to be in a state for functionality/restoration until after the meds used are completed. From the Bhasin study above it will clearly take 4+ weeks for AAS clearance yet by that time the example has stopped all SERM. Thus, an almost guarantee for ASIH.
[Note: One reason to not use AI for restoration is the T level obtained with AI are from both increased synthesis and decreased degradation. The goal for restoration is the return of synthesis capability the dual effects might cause confusion.]
Future Direction/Research
It is time for the medical community to act responsibly, intelligently, and forcefully and take control of the medical care for individuals. Time has pretty much stood still since the publication of my book on ASIH. [70] Maybe, just maybe, there is the beginning of some sense in the treatment of AAS users. In a prepublication draft, the authors state the need “to study long-term health effects of AAS and treatment strategies, and to reform regulations to stem the epidemics of AAS use.” Also, apparently the long-held notion of AAS addiction/dependency by a select few has given way to the problem of “AAS-withdrawal hypogonadism.”] [71]
At the very minimum the following research areas demand investigation:
- A does-response study on AAS and HPTA normalization. Clinical investigations regarding AAS (type, dose, duration, etc.) to development of ASIH (severity of signs & symptoms, duration, HPTA normalization);
- Clinical investigations on medical treatments (prevent, eliminate, or minimize) for ASIH; and
- Investigations on the development of protocols or programs to effect positive body composition changes without the attendant consequences of ASIH, including but not limited to obesity, COPD, CKD, HIV, and sarcopenia/frailty.
- A sensible, rational, and logical discussion of the ‘addictive’ nature of androgens with a strong reference to and inclusion of the signs and symptoms of hypogonadism as a mask to the perception of abuse or addiction. Although, the recent [prepublication] paper shows that the concept of AAS addiction/dependency has “evolved” to “AAS-withdrawal hypogonadism.”
- Street C, Scally MC. Pharmaceutical intervention of anabolic steroid induced hypogonadism – our success at restoration of the HPG axis. Med Sci Sports Exerc 2000;32(Suppl. 5).
- Scally MC, Street C, Hodge A. Androgen induced hypogonadotropic hypogonadism: treatment protocol involving combined drug therapy. The Endocrine Society 2001 Annual Meeting. Denver, CO [Abstract].
- Vergel N, Hodge AL, Scally MC. HPGA normalization protocol after androgen treatment. In: 4th international workshop on adverse drug reactions and lipodystrophy in HIV. Antiviral Therapy 2002;7:L53.
- Scally MC, Kovacs JA, Gathe JC, Hodge AL. Uncontrolled case study of medical treatment for elimination of hypogonadism after androgen cessation in an HIV+ male with secondary polycythemia treated 2 years continuously with testosterone. Endocrine Practice 2003;9(Suppl. 1).
- Park HJ. Anabolic steroid-induced hypogonadism: a challenge for clinicians. Journal of exercise rehabilitation 2018;14:2-3.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5833963/
- Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril.http://www.sciencedirect.com/science/article/pii/S001502821400140X
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism–towards a unified hypothesis of anabolic steroid action. Med Hypotheses 2009;72(6):723-8.http://www.medical-hypotheses.com/article/S0306-9877(09)00052-8/abstract
- Nangia AK. Anabolic steroid abuse: a paradox of manliness. Fertil Steril.http://www.fertstert.org/article/S0015-0282(14)00187-3/fulltext
- Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab 2001;86:5108-17.https://academic.oup.com/jcem/article/86/11/5108/2849115
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem 1997;43:1262-79.http://clinchem.aaccjnls.org/content/43/7/1262.long
- Wilson JD. Androgen abuse by athletes. Endocr Rev 1988;9:181-99.https://academic.oup.com/edrv/article-abstract/9/2/181/2548884
- Liu PY, Veldhuis JD. Chapter 12 – Hypothalamo-Pituitary Unit, Testis, and Male Accessory Organs. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe’s Reproductive Endocrinology (Eighth Edition). 2019:285-300.e8.https://www.sciencedirect.com/science/article/pii/B9780323479127000123
- Finkelstein JS, Whitcomb RW, O’Dea LS, Longcope C, Schoenfeld DA, Crowley WF, Jr. Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. The Journal of clinical endocrinology and metabolism 1991;73:609-20.https://academic.oup.com/jcem/article-abstract/73/3/609/2652816
- Finkelstein JS, O’Dea LS, Whitcomb RW, Crowley WF, Jr. Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. The Journal of clinical endocrinology and metabolism 1991;73:621-8.https://academic.oup.com/jcem/article-abstract/73/3/621/2652829
- Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-h endogenously driven and acute exogenous gonadotropin-releasing hormone-stimulated luteinizing hormone and follicle-stimulating hormone secretion in young men. J Clin Endocrinol Metab 2001;86:2600–6.https://academic.oup.com/jcem/article/86/6/2600/2849012
- Kilshaw BH, Harkness RA, Hobson BM, Smith AW. The effects of large doses of the anabolic steroid, methandrostenolone, on an athlete. Clin Endocrinol (Oxf) 1975;4:537-41.https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2265.1975.tb01566.x
- Holma P, Adlercreutz H. Effect of an anabolic steroid (metandienon) on plasma LH-FSH, and testosterone and on the response to intravenous administration of LRH. Acta Endocrinol (Copenh) 1976;83:856-64. Similar results of suppressed gonadotropins have been found in patients supplementing solely testosterone.https://www.ncbi.nlm.nih.gov/pubmed/793272
- Marynick SP, Loriaux DL, Sherins RJ, Pita JC Jr, Lipsett MB. Evidence that Testosterone can Suppress Pituitary Gonadotropin Secretion Independently of Peripheral Aromatization. Journal of Clinical Endocrinology and Metabolism. 1979;49(3):396-98.https://academic.oup.com/jcem/article-abstract/49/3/396/2679325
- Clerico A, Ferdeghini M, Palombo C, et al. Effect of anabolic treatment on the serum levels of gonadotropins, testosterone, prolactin, thyroid hormones and myoglobin of male athletes under physical training. J Nucl Med Allied Sci 1981;25:79-88. In 1981, Clerico et al found a dramatic suppression of serum gonadotropin and testosterone levels in athletes given methandrostenelone. The serum testosterone levels did not return to normal even after the gonadotropin levels returned to normal.https://www.ncbi.nlm.nih.gov/pubmed/6796662
- Bijlsma JW, Duursma SA, Thijssen JH, Huber O. Influence of nandrolondecanoate on the pituitary-gonadal axis in males. Acta Endocrinol (Copenh) 1982;101:108-12. In 1982, there was publication of the HPTA suppressive effects of nandrolone. During a pilot study regarding the possible beneficial effect of the anabolic steroid nandrolone decanoate on bone metabolism in patients with rheumatoid arthritis, study findings include a significant decrease in the serum levels of testosterone. This study demonstrates HPTA recovery was incomplete three months after cessation of nandrolone decanoate administration.https://www.ncbi.nlm.nih.gov/pubmed/6812344
- Small M, Beastall GH, Semple CG, Cowan RA, Forbes CD. Alteration of hormone levels in normal males given the anabolic steroid stanozolol. Clin Endocrinol (Oxf) 1984;21:49-55.Administering a course of the oral (C17?-alkyl derivates) anabolic steroid stanozolol in a clinical dose for 14-day resulted in a marked reduction in serum testosterone levels accompanied by reductions in LH levels. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2265.1984.tb00135.x
- Ruokonen A, Alen M, Bolton N, Vihko R. Response of serum testosterone and its precursor steroids, SHBG and CBG to anabolic steroid and testosterone self-administration in man. J Steroid Biochem 1985;23:33-8. Alen M, Reinila M, Vihko R. Response of serum hormones to androgen administration in power athletes. Med Sci Sports Exerc 1985;17:354-9. In 1985, studies on the influence 26 weeks of self-administration of anabolic steroids and a follow-up period of 16 weeks after drug withdrawal showed a major decrease in serum FSH and LH concentrations that returned to control levels following drug withdrawal. However, serum testosterone concentrations stayed at low levels during this follow-up period, indicating long-lasting impairment of testicular endocrine function.https://www.sciencedirect.com/science/article/pii/0022473185902572
- Alen M, Hakkinen K. Physical health and fitness of an elite bodybuilder during 1 year of self-administration of testosterone and anabolic steroids: a case study. Int J Sports Med 1985;6:24-9.In a reported study of an elite athlete, self-administering AAS (actually 53 mg/day) for one year showed after AAS cessation low LH, FSH, and T levels. The author’s conclusion was AAS administration affects the function of the pituitary and leads to long-lasting impairment of testicular endocrine function. https://www.ncbi.nlm.nih.gov/pubmed/3921472
- Maeda Y, Nakanishi T, Ozawa K, et al. Anabolic steroid-associated hypogonadism in male hemodialysis patients. Clin Nephrol 1989;32:198-201.Likewise, in 1989, there was reporting of anabolic steroid-associated hypogonadism to have occurred in hemodialysis patients administered nandrolone decanoate. Twenty-three patients receiving anabolic steroids showed significantly lower testosterone values than did patients without anabolic steroid administration. The authors warned that anabolic steroid administration is a possible cause for uremic hypogonadism. https://www.ncbi.nlm.nih.gov/pubmed/2805460
- Jarow JP and Lipshultz LI. Anabolic Steroid-Induced Hypogonadotropic Hypogonadism. American Journal of Sports Medicine. 1990 Jul-Aug; 18(4): 429-431.Case report studies discussed a 36-year old male competitive bodybuilder and a 39-year old father, each using various AAS regimens over extended periods of time, who showed a blunted response to GnRH stimulation tests. http://journals.sagepub.com/doi/abs/10.1177/036354659001800417
- Forbes, G. B., Porta, C. R., Herr, B. E., & Griggs, R. C. (1992). Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA, 267(3), 397-399.The sequence of changes in body composition induced by testosterone and reversal of changes after cessation was studied in 1992. Testosterone treatment produced a progressive increase in lean body mass and a progressive decrease in body fat. After the testosterone was stopped a period of hypogonadism ensued and the body composition reverted slowly back to normal. https://jamanetwork.com/journals/jama/article-abstract/394576
- Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab 1999;84:2705-11.Administration of the oral anabolic steroid oxandrolone (5 days at 15 mg/day) resulted in a significant reduction of serum total testosterone and free testosterone concentrations compared to baseline. https://academic.oup.com/jcem/article/84/8/2705/2864236
- Urhausen A, Torsten A, Wilfried, K. Reversibility of the effects on blood cells, lipids, liver function and hormones in former anabolic-androgenic steroid abusers. J Steroid Biochem Mol Biol 2003;84:369-75.In 2003, a retrospective study examined the effects of illicit AAS on a population in which the mean time off steroids was 43 months with the minimum length of time 1 year and the maximum 10 years. The study found 100% of the individuals to have HPTA dysfunction, 13/15 ex-AAS users were in the lower 20 percent of the normal reference range for testosterone and 2/15 were below the normal range (345-864-ng/dL) with 259-ng/dL and 190-ng/dL, respectively. https://www.sciencedirect.com/science/article/pii/S0960076003001055
- Gazvani MR, Buckett W, Luckas MJ, Aird IA, Hipkin LJ, Lewis-Jones DI. Conservative management of azoospermia following steroid abuse. Hum Reprod 1997;12:1706-8.Gazvani et al. followed a small series of individuals treated for infertility. Conservative management consisted of discontinuation of the offending steroid(s) and an end-point of normal semen density. The time intervals to normal spermatogenesis were from 9-22 months. https://www.ncbi.nlm.nih.gov/pubmed/9308797
- Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil Steril 1989;52:1041-7.https://www.fertstert.org/article/S0015-0282(16)53172-0/pdf
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Hormonal Male Contraception Summit Group. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis.Lancet 2006;367:1412–20. https://www.sciencedirect.com/science/article/pii/S0140673606686145
- Pena JE, Thornton MH, Jr., Sauer MV. Reversible azoospermia: anabolic steroids may profoundly affect human immunodeficiency virus-seropositive men undergoing assisted reproduction. Obstet Gynecol 2003;101:1073-5.https://journals.lww.com/greenjournal/abstract/2003/05001/reversible_azoospermia__anabolic_steroids_may.10.aspx
- Miner JN, Chang W, Chapman MS, et al. An Orally Active Selective Androgen Receptor Modulator Is Efficacious on Bone, Muscle, and Sex Function with Reduced Impact on Prostate. Endocrinology 2007;148(1):363-73.https://academic.oup.com/endo/article/148/1/363/2501521
- Gao W, Dalton JT. Ockham’s razor and selective androgen receptor modulators (SARMs): are we overlooking the role of 5{alpha}-reductase? Mol Interv 2007;7(1):10–3.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2040232/
- Basaria S, Collins L, Dillon EL, et al. The Safety, Pharmacokinetics, and Effects of LGD-4033, a Novel Nonsteroidal Oral, Selective Androgen Receptor Modulator, in Healthy Young Men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences.https://academic.oup.com/biomedgerontology/article/68/1/87/548321
- Viking Therapeutics http://www.vikingtherapeutics.com/pipeline/vk5211/
- Karavolos S, Reynolds M, Panagiotopoulou N, McEleny K, Scally M, Quinton R. Male central hypogonadism secondary to exogenous androgens: a review of the drugs and protocols highlighted by the online community of users for prevention and/or mitigation of adverse effects. Clin Endocrinol (Oxf) 2015;82:624-32.https://onlinelibrary.wiley.com/doi/full/10.1111/cen.12641
- Casarini L, Santi D, Brigante G, Simoni M. Two hormones for one receptor: evolution, biochemistry, actions and pathophysiology of LH and hCG. Endocrine reviews 2018.http://dx.doi.org/10.1210/er.2018-00065
- Stahl PJ. Recovery of spermatogenesis after hormone therapy: what to expect and when to expect it. Fertility and Sterility 2017;107:338-9.http://www.sciencedirect.com/science/article/pii/S0015028216630686
- Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertility and Sterility 2003;79:1659-61.https://doi.org/10.1016/S0015-0282(03)00365-0
- Kohn TP, Louis MR, Pickett SM, et al. Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertility and Sterility 2017;107:351-7.e1.http://www.sciencedirect.com/science/article/pii/S0015028216629199
- Gill GV. Anabolic steroid induced hypogonadism treated with human chorionic gonadotropin. Postgraduate Medical Journal. 1998;74(867):45-46.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2360778/
- Gries JM, Munafo A, Porchet HC, Verotta D. Down-regulation models and modeling of testosterone production induced by recombinant human choriogonadotropin. J Pharmacol Exp Ther 1999;289(1):371-7. http://jpet.aspetjournals.org/content/289/1/371.full
- Ulloa-Aguirre A, Lira-Albarrán S. Chapter Four – Clinical Applications of Gonadotropins in the Male. In: Kumar TR, ed. Progress in Molecular Biology and Translational Science: Academic Press; 2016:121-74.https://www.sciencedirect.com/science/article/pii/S1877117316300552
- Raven G, de Jong FH, Kaufman J-M, de Ronde W. In Men, Peripheral Estradiol Levels Directly Reflect the Action of Estrogens at the Hypothalamo-Pituitary Level to Inhibit Gonadotropin Secretion. Journal of Clinical Endocrinology & Metabolism 2006;91(9):3324-8. http://jcem.endojournals.org/content/91/9/3324.full Local aromatization of testosterone in the hypothalamo-pituitary compartment is not a prerequisite for expression of the inhibitory action of estrogens on gonadotropin secretion in men. Peripheral estradiol levels directly reflect the inhibitory tone exerted by estrogens on gonadotropin release and are a major determinant of peripheral testosterone, LH, and FSH levels.
- Birzniece V, Sata A, Sutanto S, Ho KKY. Neuroendocrine Regulation of Growth Hormone and Androgen Axes by Selective Estrogen Receptor Modulators in Healthy Men. J Clin Endocrinol Metab. http://jcem.endojournals.org/content/95/12/5443.full Tamoxifen, but not raloxifene, reduces IGF-I levels. Both SERMs stimulate the gonadal axis, with tamoxifen imparting a greater effect. We conclude that in therapeutic doses, raloxifene perturbs the GH and gonadal axes to a lesser degree than tamoxifen.
- Farmakiotis D, Farmakis C, Rousso D, Kourtis A, Katsikis I, Panidis D. The beneficial effects of toremifene administration on the hypothalamic-pituitary-testicular axis and sperm parameters in men with idiopathic oligozoospermia. Fertil Steril 2007;88(4):847-53.http://www.fertstert.org/article/S0015-0282(07)00070-2/abstract Toremifene administration for a period of 3 months in men with idiopathic oligozoospermia is associated with significant improvements of sperm count, motility, and morphology, mediated by increased gonadotropin secretion and possibly a direct beneficial effect of toremifene on the testes. The above findings are also indicative of a better testicular exocrine (improved sperm parameters) response to treatment in men whose partners achieved pregnancy compared with those who did not. Further randomized, placebo-controlled trials should be conducted to determine whether this particular selective estrogen receptor modulator can be useful as an initial approach in men with oligozoospermia.
- Tsourdi E, Kourtis A, Farmakiotis D, Katsikis I, Salmas M, Panidis D. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertil Steril 2009;91(4 Suppl):1427-30.http://www.fertstert.org/article/S0015-0282(08)01280-6/abstract This study evaluates, compares, and contrasts the effects of three selective estrogen receptor modulators (SERMs), namely, tamoxifen, toremifene, and raloxifene, on the hypothalamic-pituitary-testicular axis in 284 consecutive subfertile men with idiopathic oligozoospermia using three therapeutic protocols: [1] tamoxifen, 20 mg, once daily (n = 94); [2] toremifene, 60 mg, once daily (n = 99); and [3] raloxifene, 60 mg, once daily (n = 91). The antiestrogenic effects of SERMs at the hypothalamic level result in a statistically significant increase of gonadotropin levels, which is more marked for tamoxifen and toremifene compared with raloxifene.
- Spijkstra JJ, Spinder T, Gooren L, and van Kessel H. Divergent effects of the antiestrogen tamoxifen and of estrogens on luteinizing hormone (LH) pulse frequency, but not on basal LH levels and LH pulse amplitude in men. J Clin Endocrinol Metab 1988;66:355-60.https://academic.oup.com/jcem/article-abstract/66/2/355/2653855
- Bickelman C, Ferries L, Eaton RP. Impotence related to anabolic steroid use in a body builder. Response to clomiphene citrate. West J Med 1995;162:158–60.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1022657/
- Burge MR, Lanzi RA, Skarda ST, and Eaton RP. Idiopathic hypogonadotropic hypogonadism in a male runner is reversed by clomiphene citrate. Fertil Steril 1997;67:783-5.https://www.fertstert.org/article/S0015-0282(97)81384-2/pdf
- Guay AT, Bansal S, and Heatley GJ. Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab 1995;80:3546-52.https://academic.oup.com/jcem/article-abstract/80/12/3546/2649884
- Tan RS and Vasudevan D. Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil Steril 2003;79:203-5.https://www.sciencedirect.com/science/article/pii/S0015028202045508
- Vermeulen A, Comhaire F. Hormonal effects of an antiestrogen, tamoxifen, in normal and oligospermic men. Fertil Steril 1978;29:320–7.https://www.fertstert.org/article/S0015-0282(16)43160-2/abstract
- Ross LS, Kandel GL, Prinz LM, Auletta F.Clomiphene Treatment of the Idiopathic Hypofertile Male: High-Dose, Alternate-Day Therapy. Fertility and Sterility. 1980;33(6):618-23. https://www.fertstert.org/article/S0015-0282(16)44775-8/pdf
- Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10(6):1628-35.http://onlinelibrary.wiley.com/doi/10.1111/jsm.12116/abstract
- Wiehle RD, Fontenot GK, Wike J, Hsu K, Nydell J, et al. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertil Steril. 2014;102(3):720-7.http://www.fertstert.org/article/S0015-0282(14)00537-8/abstract
- Miller WR, Bartlett J, Brodie AMH, et al. Aromatase Inhibitors: Are There Differences Between Steroidal and Nonsteroidal Aromatase Inhibitors and Do They Matter? Oncologist 2008;13(8):829-37.http://theoncologist.alphamedpress.org/content/13/8/829.full
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 2009;94(12):4785-92.http://jcem.endojournals.org/content/94/12/4785.long
- Burnett-Bowie SA, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2008.http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2265.2008.03327.x/abstract Anastrozole administration normalized androgen production in older hypogonadal men and decreased estradiol production modestly. These alterations did not improve body composition or strength.
- de Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab 2005;7(3):211-5.http://onlinelibrary.wiley.com/doi/10.1111/j.1463-1326.2004.00397.x/full Short-term Letrozole treatment normalized serum testosterone levels in all obese men. The clinical significance of this intervention remains to be established in controlled, long-term studies.
- Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab 2004;89(3):1174-80.http://jcem.endojournals.org/content/89/3/1174.long These data demonstrate that aromatase inhibition increases serum bioavailable and total testosterone levels to the youthful normal range in older men with mild hypogonadism. Serum estradiol levels decrease modestly but remain within the normal male range. The physiological consequences of these changes remain to be determined.
- Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. European Journal of Endocrinology 2008;158(5):741-7.http://www.eje-online.org/content/158/5/741.full Letrozole 2.5?mg once a week produced a sustained normalization of serum total testosterone in obese men with IHH. However, free testosterone frequently rose to supraphysiological levels. Therefore, a starting dose <2.5?mg once a week is recommended.
- T’Sjoen GG, Giagulli VA, Delva H, Crabbe P, De Bacquer D, Kaufman J-M. Comparative Assessment in Young and Elderly Men of the Gonadotropin Response to Aromatase Inhibition. Journal of Clinical Endocrinology & Metabolism 2005;90(10):5717-22. http://jcem.endojournals.org/content/90/10/5717.full Aromatase inhibition markedly increased basal LH and T levels and the LH response to GnRH in both young and elderly men. The observation of similar to greater LH responses in the young compared with the elderly does not support the hypothesis that increased restraining of LH secretion by endogenous estrogens is instrumental in age-related decline of Leydig cell function.
- [Triptorelin] Pirola I, Cappelli C, Delbarba A, et al. Anabolic steroids purchased on the Internet as a cause of prolonged hypogonadotropic hypogonadism. Fertil Steril 2010;94(6):2331 e1-3. http://www.fertstert.org/article/S0015-0282(10)00503-0/abstract Triptorelin is a potent synthetic long-acting gonadotropin releasing hormone (GnRH) agonist most commonly associated with prostate cancer treatment. Triptorelin reversibly represses gonadotropin secretion. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=75df146d-f7a1-424e-b308-46389150ecd6 Administration used small doses [100 mcg] in comparison to those used for prostate cancer.
- [LH-RH] van Breda E, Keizer HA, Kuipers H, Wolffenbuttel BH. Androgenic anabolic steroid use and severe hypothalamic-pituitary dysfunction: a case study. Int J Sports Med 2003;24(3):195-6. https://www.thieme-connect.com/DOI/DOI?10.1055/s-2003-39089 In order to regain normal hypothalamic-pituitary function, supraphysiological doses of 200 ?g LH-RH should be considered when the physiological challenge test with LH-RH (50 ?g) fails to show an acceptable response. Gonadorelin is a decapeptide. Gonadorelin is the hypothalamic releasing factor responsible for the release of gonadotropins (e.g., LH, FSH) from the anterior pituitary. Synthetic gonadorelin is physiologically and chemically identical to the endogenous bovine hypothalamic releasing factor. https://pubchem.ncbi.nlm.nih.gov/compound/36523 /https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0b4cad4e-df2a-4192-aee1-5d75c756632a
- [Buserelin] Iwamoto H, Yoshida A, Suzuki H, Tanaka M, Watanabe N, Nakamura T. A man with hypogonadotropic hypogonadism successfully treated with nasal administration of the low-dose gonadotropin-releasing hormone analog buserelin. Fertil Steril 2009;92(3):1169 e1-3. http://www.fertstert.org/article/S0015-0282(09)01228-X/abstract GnRH Analogue, Buserelin is a synthetic analogue of gonadotropin-releasing hormone (GnRH), which is about 20 times more potent than the native hormone and is more resistant to proteolysis. It binds with high affinity to the GnRH receptor on anterior pituitary cells where it acts as an agonist. Acutely, triptorelin and buserelin stimulates the secretion of LH and FSH from the anterior pituitary, but prolonged, continuous exposure leads to inhibition of secretion by desensitization of the pituitary gonadotropes. https://pubchem.ncbi.nlm.nih.gov/compound/buserelin The patient’s laboratory examination showed low serum levels of gonadotropins and testosterone. After being diagnosed with hypogonadotropic hypogonadism, 15 ?g of buserelin acetate spray was administrated in each nostril three times a day (total: 90 ?g/day). This therapy improved semen parameters and serum gonadotropin and testosterone levels. After approximately 1 year of this treatment, the patient’s serum gonadotropin and testosterone levels remained in the normal range and semen analysis showed normozoospermia.
- Muram D, Ni X. Hypogonadal Men: The Contribution Of Endogenous Testosterone Production. ENDO 2013. https://endo.confex.com/endo/2013endo/webprogram/Paper4981.html
- Bhasin S, Storer TW, Berman N, et al. The Effects of Supraphysiologic Doses of Testosterone on Muscle Size and Strength in Normal Men. N Engl J Med 1996;335(1):1-7. http://www.nejm.org/doi/full/10.1056/NEJM199607043350101
- Scally M.Anabolic Steroids – A Question of Muscle: Human Subject Abuses in Anabolic Steroid. Available at http://asih.net/_scally_anabolic%20steroids%20-%20a%20question%20of%20muscle.pdf
- Goldman AL, Pope JHG, Bhasin S. The Health Threat Posed by the Hidden Epidemic of Anabolic Steroid Use and Body Image Disorders Among Young Men. The Journal of Clinical Endocrinology & Metabolism 2018:jc.2018-01706-jc.2018-. http://dx.doi.org/10.1210/jc.2018-01706


